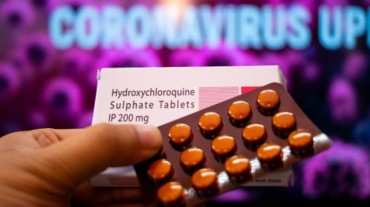
Not too long ago, hydroxychloroquine had seemed like a ‘life-saver’ when it came to the treatment of covid-19. Shortly after though, the antimalarial drug’s use for SARS-CoV-2 cases was put into question. And now, after intensive research, it has been found it comes with massive health risks, because of which WHO has temporarily suspended its clinical trial.
How did the WHO decide to suspend the drug’s clinical trial?
A study published in The Lancet found that using hydroxychloroquine as a potential treatment for covid-19 actually increased the patient’s risk of heart disease and dying. How Ironic!
After realizing the major health risks using this drug posed, WHO’s chief Tedros Adhanom Ghebreyesus stated: “The Executive Group has implemented a temporary pause of the hydroxychloroquine arm within the Solidarity Trial while the safety data is reviewed by the Data Safety Monitoring Board.”
The Solidarity Trial had become a symbol of hope after hundreds of hospitals across several countries enrolled patients to test several possible treatments for the novel coronavirus. After the massive health implications it seems to bring forth, the trials using that drug were suspended as a precaution.
Many influential public figures like the US President Donald Trump supported the use of this drug for covid-19, which led to bulk buying of hydroxychloroquine by many countries. Interestingly, even Brazil’s health minister last week recommended using hydroxychloroquine, as well as the antimalarial chloroquine, to treat mild covid-19 cases.
Yet, the Lancet study found extensive scientific evidence that put these recommendations into question. It was found that both drugs, hydroxychloroquine and chloroquine, can produce potentially serious side effects, particularly heart arrhythmia.

Even after examining records of 96,000 patients across hundreds of hospitals, the research found that neither drug could yet be seen as an effective treatment of covid-19. Nonetheless, the WHO also stated that the two drugs “are accepted as generally safe for use in patients with autoimmune diseases or malaria.
The takeaway?
With the no approved treatment or vaccine for covid-19 yet, hope of citizens of the world is wavering. Even though WHO has temporarily suspended hydroxychloroquine trial, WHO chief stressed that: “The other arms of the trial are continuing.”
To ensure that we keep the covid-19 transmission rate down and a second wave doesn’t grip the earth, WHO stated that “we must continue to put in place a comprehensive strategy to ensure that we continue on a downward trajectory and that we don’t have an immediate second peak.”
We must remain conscious of the impact the virus is making and ensure that we follow all necessary guidelines to ensure that we’re safe from covid-19.
Select Topics of your interest and let us customize your feed.
PERSONALISE NOW(With inputs from AFP)
Get Latest Updates on Health News